MEASUREMENTS
IN SCIENCE

Fig. 1 - The seven base SI units and their symbols.
SI BASE UNITS
There are seven standard 'base' or 'fundamental' SI units (see Fig. 1); all other SI units are derived from these base units.
Standard international units are typically globally used and recognized units assigned to certain types of quantities. These SI units also have recognized symbols used to denote the unit. This is different from mathematical symbols used to denote the type of quantity; for example, an equation may denote the variable of time with 't', but when you solve the equation for 't', the answer will be in seconds, 's'.
Examples of SI units include:
-
Length - meters (m)
-
Centimeters (cm) = 1/100 * meters
-
Millimeters (mm) = 1/1000 * meters
-
Kilometers (km) = 1000 * meters
-
-
Mass - kilograms (kg)
-
Grams (g) = 1/1000 * kilograms
-
Milligrams (mg) = 1/1000000 * kilograms
-
-
Time - seconds (s)
-
Minutes (m) = 60 * seconds
-
Hours (hr) = 3600 * seconds
-
-
Volume (solids) - centimeters-cubed (cm^3)
-
Volume (liquids)- liters (l)
-
Liters (l) = 1000 * cm^3
-
Milliliters (ml) = 1/1000 * liters
-
SIGNIFICANT FIGURES
All non-zero digits are significant; trailing zeroes on the right of decimals are significant; zeroes between significant figures are also significant. Moreover, the 'n' number in the standard form/scientific notation of numbers will be significant according to the general rules of significance, but the '10' and its index will not count as a significant figure.
Non-significant figures include:
-
Zeroes trailing a whole number
-
e.g. 123000
-
an exception to this rule is that zeroes trailing on the left side of a decimal point will be significant - 120.0 has three significant figures
-
-
Zeroes leading any number
-
e.g. 042, 0.00123
-


COMPOUND UNITS
Compound units are derived from base/fundamental units and are used to increase the efficiency of varying values.
The most common example of this is a Newton (N).
1 Newton is equivalent to:
1 Kilogram x 1 Meter ÷ 1 Second x 1 Second
or
kg*m/s^2
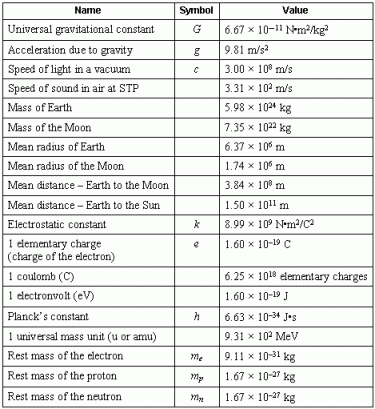
STANDARD FORM
Standard form, also referred to as scientific notation, is a method used to shorten numbers. The numbers are expressed as powers of ten; the general form of scientific notation is as follows:
N * 10^x
In this case, 'N' is a number with only one digit (unit) on the left of a decimal and two/three (or as appropriate) digits on the right of its decimal. 'x' is the power of 10 which indicates how many numbers are on the right side of the decimal of 'N'.
Examples of standard form are:
-
134 = 1.34 x 10^2
-
10,800,000 = 1.08 x 10^7
-
0.01 = 1 x 10^-2
-
0.00123 = 1.23 x 10^-3