MEASUREMENT AND DATA PROCESSING
QUALITATIVE DATA - Non-numerical data, obtained from observation.
QUANTITATIVE DATA - Numerical data, measurements taken.
Precision - How close several experimental measurements/values are to each other.
Accuracy - How close the readings/values are to the actual value.

DEPENDENT VARIABLE
WHEN PLOTTING GRAPHS
ERRORS
-
Random
-
Systematic (e.g. parallax, non-zero)
INDEPENDENT VARIABLE
-
Independent variable on x-axis, dependent variable on y-axis
-
Label axes with correct units
-
Choose an appropriate scale
-
Give an appropriate title
-
Draw line of best fit
Outliers - Points that don't fit the trend
SPECTROSCOPIC ANALYSIS OF ORGANIC COMPOUNDS
1. INDEX OF HYDROGEN DEFFICIENCY (IHD) -> Degree of unsaturation
No. of molecules of H2 that are needed to convert organic compounds to their corresponding saturated, non-cyclic molecule.
For a hydrocarbon CxHy, the IHD is given by:
IHD = (2x +2-y)/2
-
Each double bond/ring counts as IHD = 1
-
Each triple bond counts as IHD = 2
RULES
-
Oxygen and sulfer atoms in compounds don't affect IHD
-
Halogen atoms are treated like hydrogen atoms
-
For each nitrogen atom, add one carbon and one hydrogen atom to the molecular formula
1 ring + 3 double bonds
IHD = 4
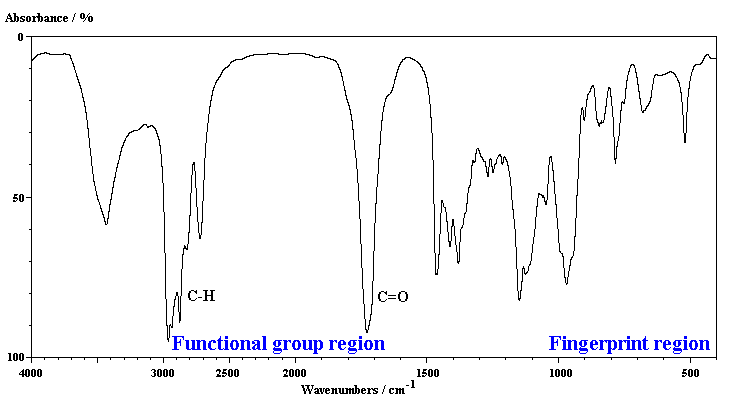
2. INFRARED (IR) SPECTROSCOPY
Used to identify the bonds present within a molecule
-
IR radiation is absorbed by certain bonds and causes them to stretch or bend.
-
Bonds connecting the atom can be viewed as behaving as tiny springs and vibrating at characteristic frequencies.

IR radiation absorbed depends upon polarity. Strong polar results in more IR radiation absorbed!
VIBRATION
Asymmetrical stretching
Asymmetrical bending
cause change in dipole moment of the molecule
Light atoms
Multiple bonds
vibrate at higher frequencies
Degree of stretching/bending depends upon strength of the bond and type of atoms bonded together.

3. MASS SPECTROMETRY (MS)
Determine Ar of an element and determine structure of an organic compound.
Molecules break up into different fragments.
Fragmentation pattern gives evidence about structure of compound
(molecular ion piece)
VIADD
4. PROTON NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY (H NMR)
-
To identify different chemical environments in a molecule that contain hydrogen atoms (or protons)
-
Atoms with nuclei that contain an ODD number of protons can spin clockwise, or counterclockwise, generating a magnetic field.
When placed in an external magnetic field, some nuclei will line up with the field, while others will line up against it.


