CHEMICAL BONDING

Elements create bonds in order to obtain stable gas configuration through the gain, loss, or sharing of electrons.
Covalent bond – Electrostatic forces of attraction between positively charged nuclei and shared pair of electrons.
Metallic bond – Electrostatic forces of attraction between positive metal ions and a sea of delocalized electrons.
Ionic bond – The electrostatic attraction between oppositely charged ions. Ionic bonds are formed by elements with an electro-negativity difference of 1.8 units or larger.
Ionic Bonding
-
Non-directional
-
Giant Ionic Lattice Structure
Valency – Combining power of an element
Coordination number – Expresses the number of ions that surround a given ion in a compound.
e.g.
Ammonium chloride NH4Cl (Both non-metals make this compound. It features two types of bonding – ionic between the ions and covalent bonding between the atoms that make up NH4.)

HOW ARE IONIC BONDS FORMED?
Complete transfer of electrons from a metallic atom to a non-metallic atom, to form oppositely charged ions which attract each other.
STRENGTH OF AN IONIC BOND
1. Charge of the ion (larger - stronger)
2. Size of the ionic radius (smaller - stronger)

MAGNESIUM CHLORIDE
.jpg)
LITHIUM NITRIDE
.jpg)
LEWIS STRUCTURE
Shows all valence electrons in a covalently bonded species.
RULES
1. Add total valence electrons
2. Draw skeletal structure
3. Use a line between each element to symbolize an electron pair
4. If not enough electrons, use double/triple bonds
5. Ions must have square brackets around them with a charge.
SODIUM OXIDE

Covalent Bonding
In covalent bonds, strength increases if:
-
bond length decreases
-
shared electrons increases
(Orbitals must be completely empty or half filled to take place in bonding)
BEN - Breaking bonds is endothermic
MEX - Making bonds is exothermic
tendency to attract a shared pair of electrons to itself.
BOND STRENGTH - How much energy is required to break the bond.
triple bond > double bond > single bond
SIGMA - Head on overlapping of orbitals
PI - Sideways overlapping
*When atomic orbitals overlap, molecular orbitals are formed.
POLAR BOND - When there is an electro-negativity difference.
(i) electronegativity difference
(ii) dipole moment (charge & distance)
is 0 when element is the same.
MOST ELECTRO-NEGATIVE: N, O, F
If molecule is symmetrical, it is not polar!
Electronegativity difference!
-
Difference of < 1.8 shows bond considered covalent
-
0.0 - 0.4 considered non polar
-
0.4 - 1.8 considered polar
-
1.8 < considered ionic

COORDINATE COVALENT BONDS - Atoms must have at least one lone pair. Electrons come from only one atom.

RESONANCE STRUCTURES

OCTET RULE - Refers to the tendency of atoms to gain a valence shell with a total of 8 electrons.
Left over electrons are shifted to the central atom (can have more than 8).
Terminal & Central Placements


COORDINATE BONDS

carbon monoxide


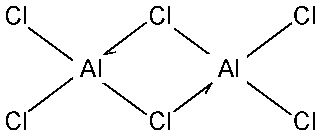
dimer of aluminum chloride
ammonium cation
VSEPR Theory (Valence Shell Electron Pair Repulsion Theory)
-
The pair of valence electrons are arranged as far apart as possible from each other.
-
Focus on central atom and see how many bond and lone pairs there are.
-
1 double/triple bond will be considered 1 bond.
1. Electron Domain Geometry - How many bond and lone pairs there are.
2. Molecular Geometry - Overall shape of the molecule





Giant Molecular Structures
Allotropes - Different forms of an element in the same physical state.
Different bonding within the structure gives rise to different physical properties.
GRAPHITE
-
Each atom is sp2 hybridized, covalently bonded to 3 others
-
Hexagons in parallel layers with bond angles of 120 degrees
-
Layers held together by Van der Waal's forces of attraction, slide over each other
-
Density: 2.26g/cm3
-
Contains one non-bonded, delocalized electron per atom
-
Bad heat conductor
-
Very high melting point, most stable allotrope
-
Non-lustrous, grey solid
-
Lubricant, pencils
DIAMOND
-
Each atom is sp3 hybridized, covalently bonded to 4 others tetrahedrally in regular repeating patterns with 109.5 bond angles
-
Hardest known natural substance
-
Density: 3.51 g/cm3
-
All electrons bonded, thus does not conduct electricity
-
Very good head conductor
-
Very high melting point
-
Brittle
-
Lustrous crystal
-
Jewelry; tools
GRAPHENE
-
Each C atom is sp2 hybridized and covalently bonded to 3 other carbons, forming hexagons with bond angle 120.
-
Two dimensional single later (honeycomb structure)
-
Density: 1.56 g/cm3
-
contains one non-bonded, delocalized electron per atom
-
Conducts electricity
-
Excellent heat conductor
-
Very high melting point, stronger than steel
-
Thinnest material to exist; almost completely transparent
-
Touch screens; performance electronics
FULLERENE (C60)
-
Each C atom is sp2 hybridized, covalently bonded in a sphere of 60 carbon atoms, consisting of 12 pentagons and 20 hexagons
-
Closed spherical cage in which each C atom is bonded to 3 others
-
Density: 1.726 g/cm3
-
Easily accepts electrons to form negative ions; semi-conductor at normal temp and pressure
-
Very low heat conductivity
-
Low melting point
-
Yellow crystaline solid - soluble in benzene
-
Nanotubes; catalysts, and lubricants
Metallic Bonding
The electrostatic forces of attraction between a lattice of positive metal ions and a sea of delocalized electrons.
STRENGTH OF A METALLIC BOND
-
No. of delocalized electrons (more - stronger)
-
Size of cation (smaller - stronger)
-
Charge of cation (larger - stronger)
PROPERTIES OF METALS
- Good electricity conductors
- Good heat conductors
- Malleable
- Ductile
- High melting point
- Shiny and lustrous (delocalized electrons reflect light!)
ALLOYS
1) Steel - Iron + carbon + XYZ
High tensile strength, tends to corrode
2) Stainless steel - Iron + nickel/chromium
High strength, corrosion resistant
3) Brass - Copper + Zinc
Plumbing
4) Bronze - Copper + Tin
Coins + Tools
5) Pewter - Tin + Antimony + Copper
Decorative objects
6) Sterling silver - Silver + copper
Jewelry + art objects
Van Der Waal's Forces (Intermolecular forces)
LONDON FORCES
-
Exists in all molecules
-
Temporary dipole moment is created due to constant shifting of electron clouds
-
This instantaneous dipole has an influence on adjacent molecules
-
Causes the positive nuclei to be attracted to the electron cloud of another molecule, and results in temporary dipoles to be created and molecules to become unsymmetrical
What affects magnitude?
- Number of electrons
- Size (volume) of electron cloud
- Shape of molecules
DIPOLE-DIPOLE FORCES
-
Exists in all polar molecules with a permanent dipole moment
-
e.g. HF, ICl, HCl, CH3, CHO
-
Attraction between the positive end of one permanent dipole and the negative end of another permanent dipole on an adjacent molecule.
HYDROGEN BONDING
-
Between most electronegative elements O, N, F and H (e.g. O-H, N-H, F-H)
-
Examples: ammonia (NH3), hydrogen fluoride ions (HF), water (H2O), (CH3)2O
-
Strongest type of intermolecular forces of attraction
DIPOLE-INDUCED DIPOLE
-
A weak attraction when a polar molecule induces a dipole in an atom or in a non-polar molecule by distrubing thr arrangement of electrons in non-polar species.
STRENGTH OF INTERMOLECULAR FORCES!
HYDROGEN BONDS > DIPOLE-DIPOLE > DIPOLE-INDUCED DIPOLE > LONDON FORCES