CARBOHYDRATES, LIPIDS, AND PROTEINS
Organic & Inorganic Compounds
- Organic compounds contain carbon and are found in living things. They usually contain C-C or C-H bonds (hydrocarbons).
- Inorganic compounds do not contain carbon.
- Inorganic compounds that DO CONTAIN carbon are simple carbon compounds:
-
Oxides of carbon
-
Hydrogen carbonates
-
Carbonates of metals
Amino Acids
- There are 22 different protein making amino acids of which 20 are coded for in genetic code. Each amino acid has a unique 'R' group.
- Amino acids may be polar or non-polar, and their different properties determine their interactions and shape of the final protein.
- The different properties of amino acids depends upon their R side chain.
- An amino acid is a zwitter ion. The carboxylic acid of one amino acid joins with the amine group of another amino acid to create a di-peptide.


Methionine (First amino acid in all peptide chains as it is the first produced in transcription in ribosomes) AUG codon


Sugars

GLUCOSE (C6H12O6) - Six-carbon sugar (hexagon)

RIBOSE (C5H10O5) - Five-carbon sugar (pentagon)
*Always count carbons in a clockwise direction starting from the first sugar after the oxygen atom in the ring structure!

Fatty Acids & Glycerols
Fatty acids can be of many lengths, extended by adding CH2 units. They are an efficient storage of energy and and bond with glycerol (a simple sugar alcohol) to make triglycerides (lipids).

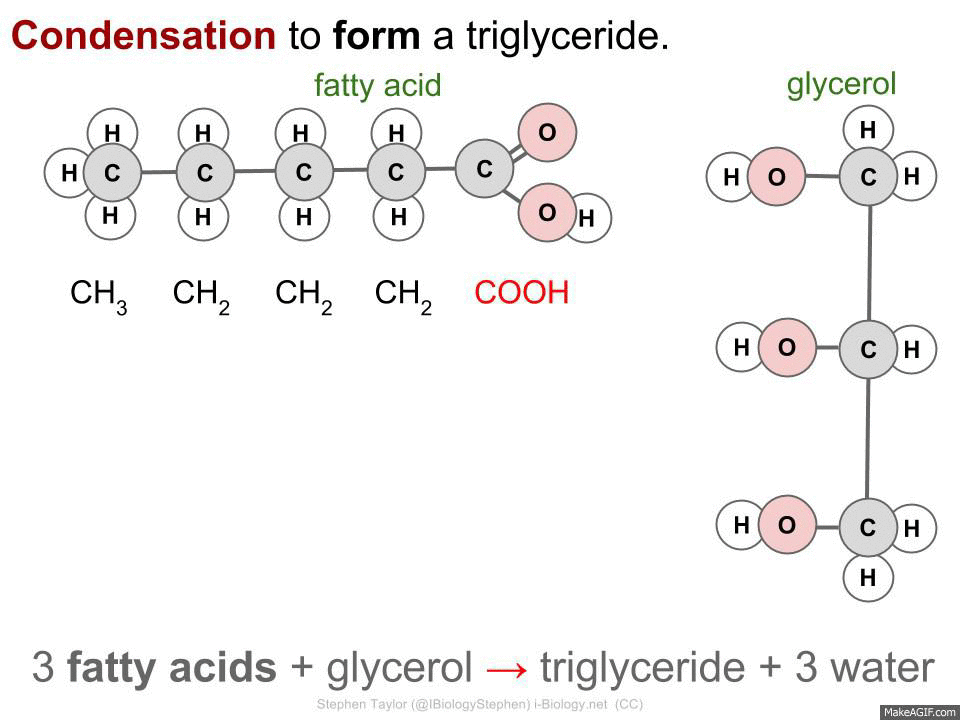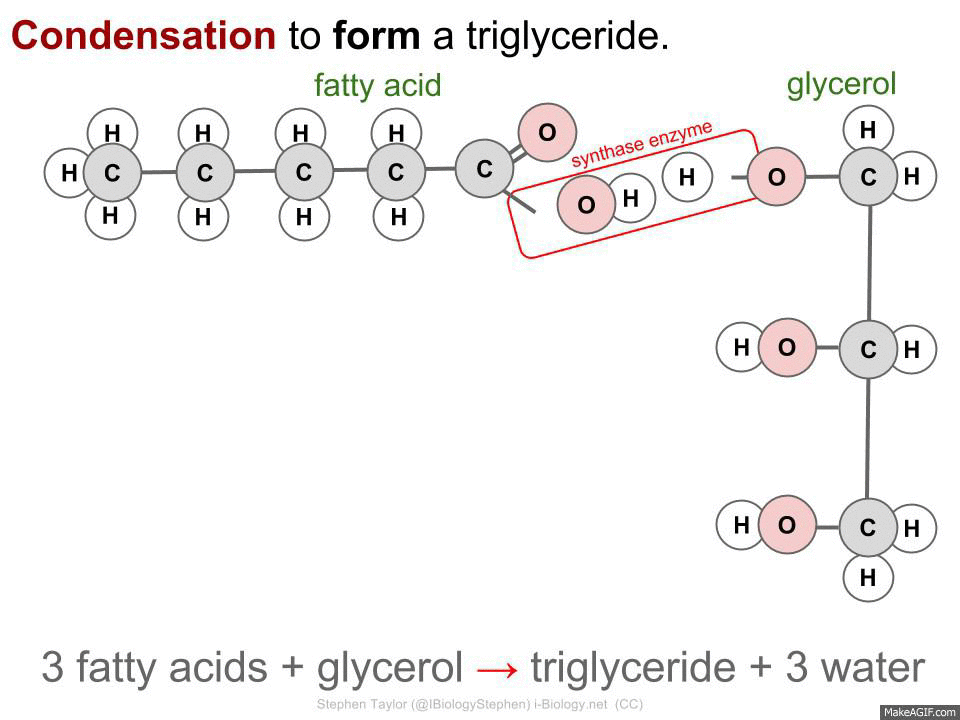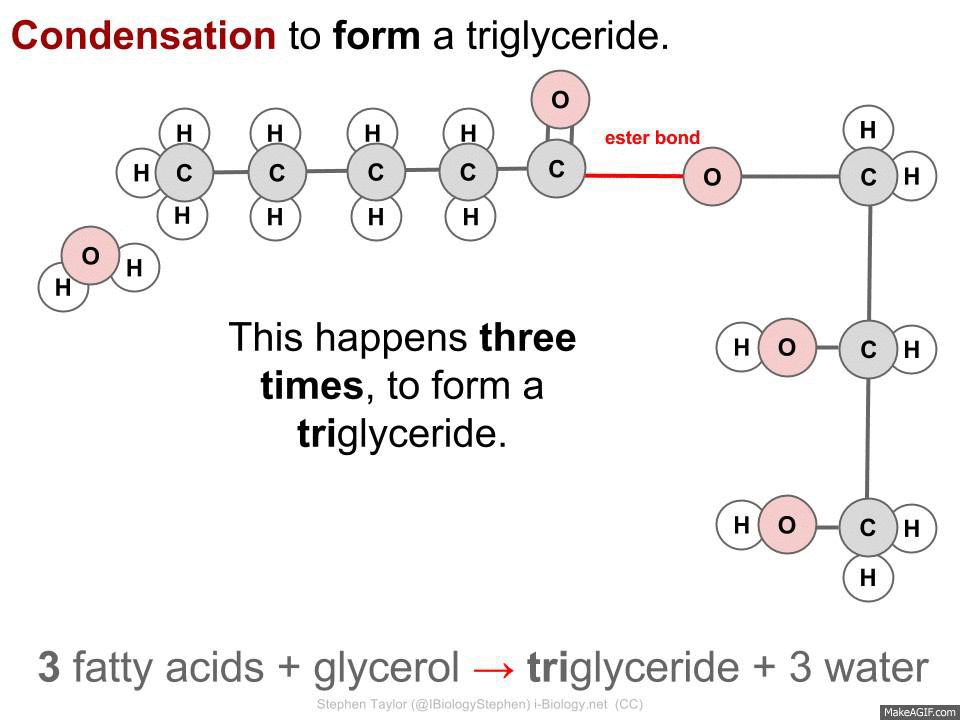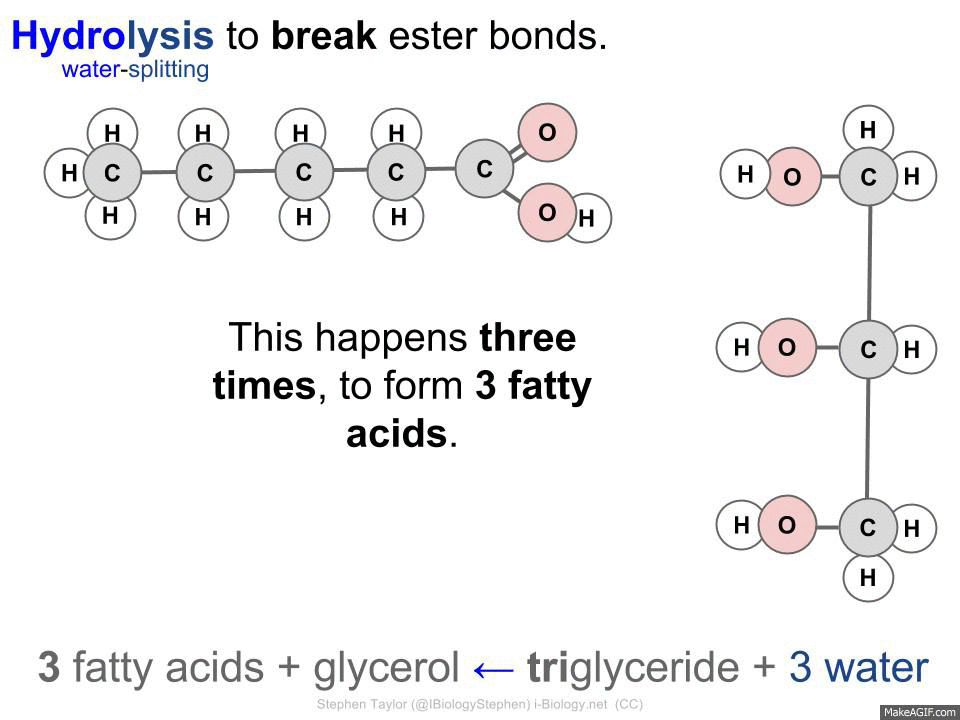


Single C-C bonds -> Saturated fats. SOLIDS
Double C=C bonds -> Unsaturated fats (kink in the chain, pulls everything closer. LIQUIDS
- Polyunsaturated (>1 C=C bond
- Monounsaturated (1 C=C bond)
MONOSACCHARIDES
-> Small easily absorbed sugars (fast release for respiration)
-
GLUCOSE (animal)
-
GALACTOSE (animal)
-
FRUCTOSE (plant)
DISACCHARIDES
-> Quickly digested into their monosaccharides
-
LACTOSE (animal) [glucose + galactose] Quickly digested, found in milk
-
MALTOSE (animal) [glucose + glucose] Quick store for energy, broken down from starch
-
SUCROSE (plants) [glucose + fructose] Soluble but unreactive, can be transported around plant in phloem.
POLYSACCHARIDES
-> Insoluble storage molecules
-
GLYCOGEN (animal) Excess sugar converted by insulin for storage in liver
-
CELLULOSE (plants) Structural component (cell wall)
-
STARCH (plants) Storage sugar
Making of Proteins
1. PRIMARY STRUCTURE
Simple chain of polypeptides
Polypeptides made up of amino Acids
2. SECONDARY STRUCTURE
Secondary interactions due to the different nature of amino acids (R side chains)
Alpha-Helix and Beta-Sheet formed (temporary polar bonds)
3. TERTIARY STRUCTURE
Due to R chains in amino acids
Disulfide, sulfydrol
Cystine bridge
Ionic bonds
Hydrogen bonds
4. QUATERNARY STRUCTURE
When many polypeptides join together to make a protein

HYDROLISIS - A complex molecule is broken down into simpler molecules using a water molecule.
dipeptide + water -> amino acid + amino acid
maltose + water -> glucose + glucose
triglyceride + 3 water -> 3 fatty acids + glycerol
CONDENSATION - Two simple molecules are joined together to make a complex molecule and a water molecule.
amino acid + amino acid -> dipeptide + water
glucose + glucose -> maltose + water
3 fatty acids + glycerol -> triglyceride + 3 water
Synthase - Builds up larger molecules
Hydrolase - Breaks down larger molecules
Uses of lipids in living organisms?
Energy Storage - Efficient storage mechanism for energy compared to carbohydrates (38 kJ/g of energy vs. 17kJ/g of energy).
Thermal insulation - high ability to insulate against cold, packing of fat is advantageous in cold climates.
Protection - Padding effect of fat protects vital organs against impacts.
Buoyancy - Lipids are less dense than water, large water-bound animals can float more easily.
Membranes - Non polar lipid component of the phospholipid bilayer repels water, forming membrane and protecting content of cells.
Hormones - Some hormones are lipid-based, e.g. cholesterol, testosterone, and cortisol.