ATOMIC STRUCTURE
Atom - Smallest unit of an element that can:
-
Exist independently
-
Show properties of that element
-
Take part in chemical reactions
PARTS OF AN ATOM:
1) Nucleus (protons + neutrons)
2) Shells/ Energy levels (electrons)
Particles may be:
-
molecules
-
atoms
-
ions
*** 1AMU is 1/12 the mass of a carbon atom.
SUBATOMIC PARTICLES
Protons = 1 AMU (+)
Neutrons = 1 AMU (+-)
Electrons = 1/1840 AMU (-)
1 AMU = 1.67 x 10 g
AMU - Atomic Mass Unit
-24
Shell - Imaginary path around the nucleus where electrons revolve (K,L,M,N).
ATOM
gains e
loses e
Anion (-)
Cation (+)
X
A
Z
A = Mass no./ nucleon no.
Z = Atomic number
Element - Substance that cannot be broken down into simpler substances by a chemical reaction.
Compound - When 2 or more different elements are chemically combined together in a fixed ratio.
Matter - Has mass and takes up space.
Molecule - Smallest part of an element or covalent compound.
- Molecule
- Compound
- Molecule
O
2
N
3
H
2
O
Isotopes - Atoms of the same element that have the same number of protons but different numbers of neutrons.
Isotope Enrichment - The process of concentrating a particular isotope in a sample by removing other isotopes.
Isotopes have the same chemical properties but different physical properties.
H
1
1
(0 n)
H
2
1
(1 n)
H
3
1
(2 n)
- Chemical properties are dependent on electrons (especially valence electrons).
- Physical properties are dependent on atomic mass.

impure
MATTER
pure
MIXTURE
SUBSTANCE
HOMOGENEOUS
HETEROGENEOUS
COMPOUND

COVALENT
MOLECULES
IONIC
IONS
METALLIC
ELEMENT
Models of the atom over time
1) John Dalton - Dalton's Atomic Theory
- All mater was composed of atoms - cannot be divided further
- Different elements had atoms of different sizes and mass
- All compounds made of atoms in fixed ratios combinations
- Chemical reactions resulted in re-arrangement of reacting atoms
2) JJ Thompson - Plum Pudding Model & Discovery of the electron
- Atom is composed of electrons surrounded by a soup of positive charge to balance the negative charges.
3) Ernest Rutherford - Gold foil experiment & discovery of proton
- Atom has a tiny, high mass positively charged nucleus
- Many alpha particles were deflected at small angles, while others were reflected back to the alpha source (during the gold foil experiment)
4) Niels Bohr - Solar system model & where electrons orbit the nucleus
- Electrons in an atom orbit the nucleus in a stable fashion
- Electrons can only gain or lose energy by jumping from one orbit to another
5) Quantum Mechanical Model - Modern theory where electrons exist in cloud shapes called 'orbitals'
- Orbitals where it is more likely for an electron to be present


Figure 1. Shells in an atom. The distance decreases between shells as you move farther away from the nucleus. The shell closest to the nucleus has the least energy. As you move up the energy increases.

Figure 2. Subshells in an atom. Note that there are the same number of subshells in a shell as the number (n) of said shell. (If n is 3, there will be three subshells).

Subshell in an atom
Orbital in a subshell. The chances of finding an electron increases in an orbital.

Electronic Configuration

- Shells are filled from lowest energy to highest energy (s < p < d < f).
- As can be seen from the diagram on the right, s contains 2 electrons, p contains 6 electrons, d contains 10 electrons, and f contains 14 electrons. This information can be used to find the electronic configuration of various elements.
BASIC CONFIGURATION:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, 8s.
Degenerate orbitals - Having the same energy (e.g. 2Px, 2Py, 2Pz).
Atomic orbitals depend on axis.
Electronic arrangement gives the number of electrons in each main energy level (e.g. 2, 8, 8)
Electron configuration gives the number of electrons in each subshell (1s, 2s, 2p, 3s, 3p)
Figure 3. Electronic configuration
THE THREE RULES TO REMEMBER WHEN IT COMES TO ELECTRONIC CONFIGURATION!
-
Aufbau Principle - Electrons enter orbitals of lowest energy first
-
Pauli Exclusion - An orbital can only hold 2 electrons with opposite spins (one clockwise, and one counterclockwise)
-
Hund's Rule - Electrons enter orbitals singly until they have to pair up.
How electrons fill orbitals:
1. First electrons enter orbitals singly.
2. When all orbitals have been filled with one electron, the second electron enters each orbital (2 per orbital).
3. An orbital can be one of the three - completely filled, half-filled, or incompletely filled.
2p
Px Py Pz
2p
Px Py Pz
2p
Px Py Pz
HALF-FILLED
Only 1 electron in every orbital, comparatively more stable than an incompletely filled subshell.
INCOMPLETELY FILLED
1 electrons in each orbital. After this other electrons pair up. Two orbitals have 2 electrons with opposite spins. Unstable.
COMPLETELY FILLED
2 electrons in each orbital. Each orbital has 2 electrons with opposite spins.
Stable.
COMPLETELY FILLED - Very stable
HALF FILLED - Stable
INCOMPLETELY FILLED - Unstable
3 WAYS TO WRITE ELECTRONIC CONFIGURATION
1. Full electronic configuration
2. Condensed electronic configuration
3. Orbital diagram representation
- Completely filled orbitals.
1s 2s 2p 3s
- Pre-filling energy of 4s is lower than that of 3d.
- Post -filling energy of 4s increases and energy of 3d decreases.
- Electron will be removed from 4s first since it has higher energy, but will also fill 4s first.
- When writing electronic configuration, always write 3d before 4s, but make sure to fill 4s first.
PRACTICE QUESTIONS


Mass Spectrometer & Calculating Ar
Mass spectrometer - Used to measure the mass of charged particles (only positive ions are detected).
STEPS INVOLVED:
1. Vaporization - Sample is turned into gas.
2. Ionization - Electron beam provides high energy and knocks of valence electrons to produce a positively charged ion.
3. Acceleration - Ions are accelerated towards negative plates at a speed dependent on their mass.
4. Deflection - Ions passed through electromagnets causing them to bend at a certain angle (depends on mass:charge ratio [m:z ratio]).
5. Detection - Depending on the mass of each ion, the ions are deflected at different angles and detected. These results are amplified, and presented digitally in the form of a mass spectrum.
-
The isotope with the greatest mass and smallest charge is deflected the least.
-
Since charge is controlled in a mass spectrometer, only the mass changes, and the mass of the ions can be found using the m:z ratio.
-
Curve of an ion through an electromagnetic field depends on the m:z ratio.

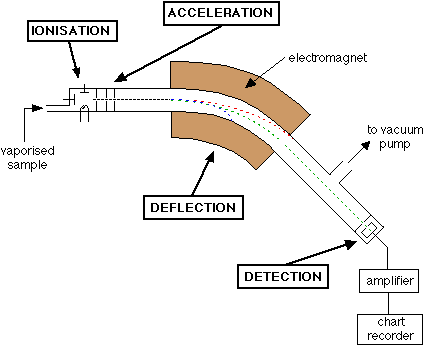
Figure 4. Mass Spectrometer Diagram
CALCULATING AR

Figure 5. Mass Spectrum of Pentane


Electromagnetic Spectrum
*Highest wavelength (lowest energy)
*Lowest frequency
RADIO
INFRA RED
ULTRAVIOLET
GAMMA
MICRO
VISIBLE
X-RAYS
(ROYGBIV)
*Lowest wavelength (highest energy)
*Highest frequency
TWO TYPES OF SPECTRUMS
-
Continuous Spectrum - Produced when a white light is passed through a prism. It shows all the colors in an unbroken sequence.
-
Line Spectrum - Produced by excited electrons as they fall to a lower energy level. Sharp lines are produced by specific frequencies of light.
-
Emission Spectrum - Colored lines on a dark background (Pure gaseous element + electrical discharge)
-
Absorption Spectrum - Black lines on colored background (Hot metal + Cloud of cold gas + Detector)
-

Figure 6. Hydrogen Spectrum
- The line emission of a hydrogen spectrum proves the existence of electrons in discrete energy levels which converge at higher energy levels.
- The colors and emission/placement of lines of each element's line spectrum is unique and different.


LAW OF CONSERVATION OF MASS
-
Equation should be balanced
-
Equal number of atoms on both sides

If solvent is water, solution is aqueous solution.
- If solvent > solute, dilute aqueous solution
- If solute > solvent, concentrated aqueous solution
GAS
SOLID
LIQUID
Sublimation/
Deposition
Vaporization/
Condensation
Melting/ Freezing
STATE CHANGE IS CAUSED BY
- Heating
- Cooling
- Pressure
Dynamic Equilibrium - When the rate of forward reactions becomes equal to the rate of backward reactions.
Latent Heat of Vaporization / Fusion
Heat used to break down the forces of attraction holding particles together
Types of Reactions
1. Combination or Synthesis
2. Decomposition
3. Single Displacement
4. Double Displacement
5. Combustion
A + B -> AB
AB -> A + B
A + BC -> AB + C
AB + CD -> AD +BC
A + O -> AO
2
2